| bit | Xplor-NIH | VMD-XPLOR |
|---|
|
| Xplor-NIH home Documentation |
Next: Syntax Up: Coordinate Restraints Previous: Example: Plane and Point
Dihedral Angle Restraints
This section shows how to restrain dihedral angles to particular values.
The functional form of the effective
energy 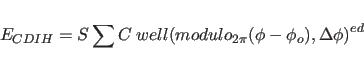 |
(7.3) |
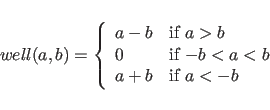 |
(7.4) |
Subsections Xplor-NIH 2025-11-07
